All Categories
History












This section provides an overview for halogen lamps as well as their applications and principles. Also, please take a look at the list of 26 halogen lamp manufacturers and their company rankings. Here are the top-ranked halogen lamp companies as of July, 2025: 1.The David Round Co., Inc., 2.Bulbrite, 3.Heraeus Holding GmbH.
Table of Contents
1976~2001: Worked at the Geological Survey of the Industrial Science and Technology Agency of the Ministry of International Trade and Industry (initially). Engaged in research on resource geology at the Hokkaido Branch.
2001~2016: Worked as an industry-academia-government collaboration coordinator at the National Institute of Advanced Industrial Science and Technology, and also served as the head of the Manufacturing Fundamental Technology Support Office.
2004~2016: R&B Park Sapporo Odori Satellite is a base to support the development of products and technologies through industry-academia-government collaboration in Hokkaido.
Currently working as a science and technology advisor.
A halogen lamp is a type of incandescent lamp that contains trace amounts of halogen elements (iodine, bromine, etc.) in addition to inert gases such as nitrogen and argon.
Halogen lamps emit light in the same way as ordinary incandescent lamps, by passing electricity through a filament inside the bulb. The filament is a thin, thread-like metal wire, most often made of tungsten, which has the highest melting point of all metals (3,422°C).
Halogen lamps are used for spotlighting on product shelves, floodlighting, car headlights, studio and stage lighting, and other applications because of their compact size, high luminance, easily adjustable light distribution (light spread), and good color rendering properties (colors are close to those seen in sunlight). However, with the spread of LED light sources, opportunities for use in lighting applications are decreasing.
Halogen lamps have been used as light sources for OHPs and slide projectors used in schools, etc. Today, LED and laser light sources are becoming the mainstream.
Light sources for spectroscopic analysis are used because they have a constant brightness over a wide range of wavelengths and little fluctuation in intensity over time.
The fact that the majority of the energy radiated is infrared tells us that halogen lamps as light sources are inefficient but excellent heaters. Therefore, halogen lamps have applications in a variety of situations requiring localized heating, such as heat retention, heat treatment, drying, and high-temperature molding of food and materials, in addition to localized heating indoors and outdoors.
The filament temperature of ordinary incandescent lamps ranges from 1,500 to 3,000°C, while that of halogen lamps is usually as high as 2,500 to 3,000°C, with special ones reaching as high as 3,300°C. Therefore, a small amount of tungsten is constantly evaporating on the surface of the filament.
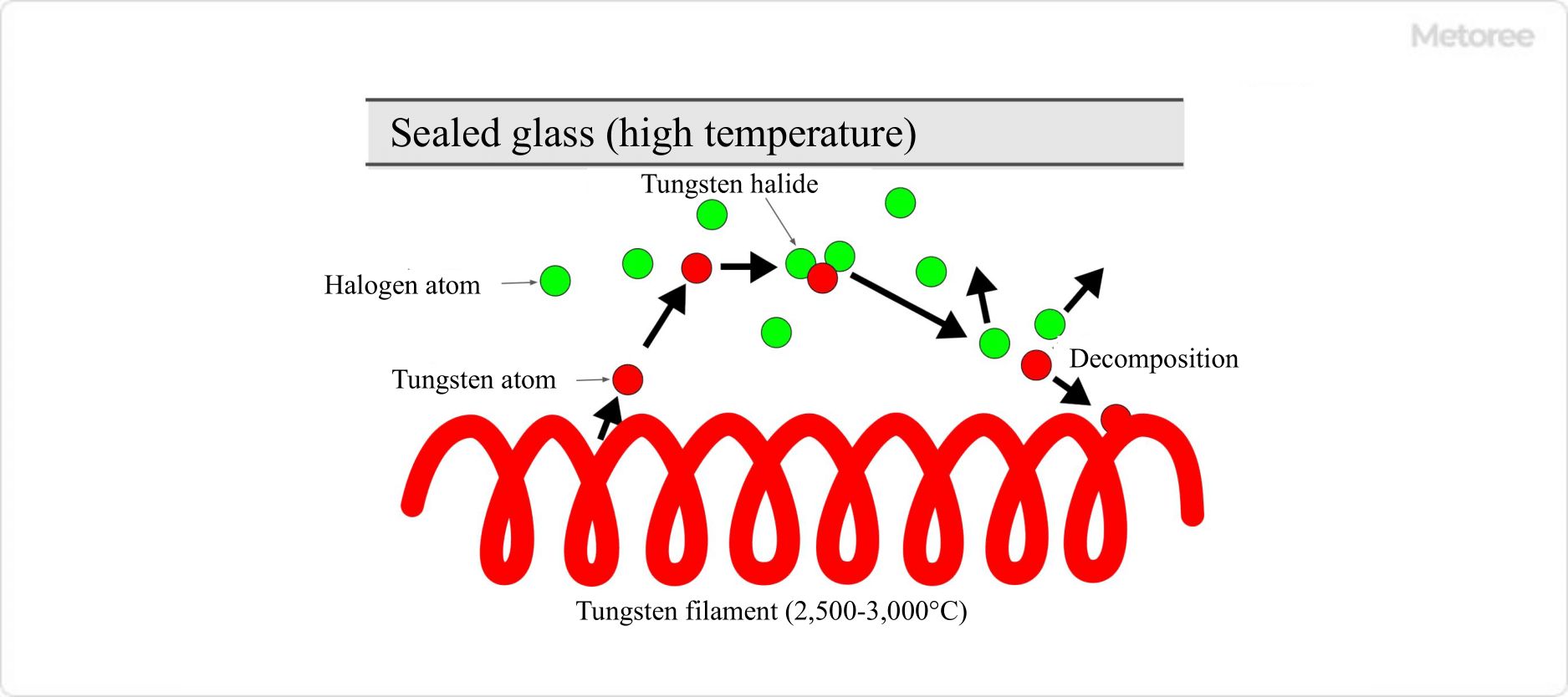
Figure 1. Halogen Cycle
To suppress the blackening phenomenon, halogen lamps contain a small amount of halogen elements along with inert gas in the bulb. In this way, if conditions such as temperature and materials are appropriate, the blackening phenomenon will not occur due to the halogen cycle that occurs in the lamp.
The halogen cycle is a phenomenon that occurs in the following manner.
The halogen cycle prevents filament wear and tungsten-induced blackening of the glass inner wall.
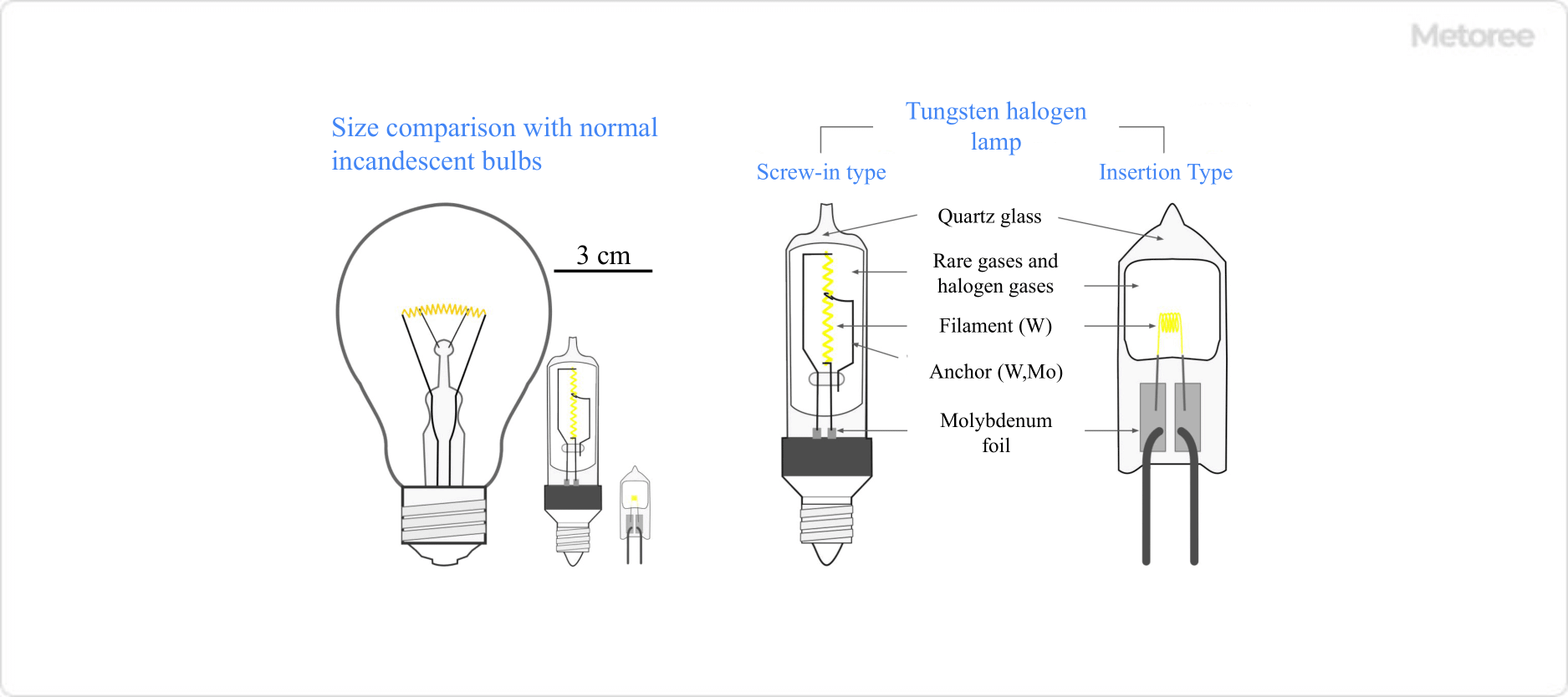
Figure 2. Incandescent and Halogen Lamps
To achieve a halogen cycle, the encapsulated glass must be kept at a high temperature. When iodine is used as a halogen gas, the glass temperature must be 170°C or higher, and when bromine is used, the glass temperature must be 250°C or higher.
Therefore, quartz glass, which can withstand high temperatures, is usually used, and molybdenum foil is used to electrically connect the inside and outside of the halogen bulb so that the inside remains airtight even at high temperatures.
In ordinary incandescent bulbs, blackening occurs when evaporated tungsten adheres to the inner wall of the bulb's glass. As the filament wears out, the luminous efficiency inevitably decreases.
This blackening phenomenon is an obstacle, making it difficult to miniaturize incandescent bulbs with high power consumption or to raise the filament temperature to higher levels to increase brightness and color temperature.
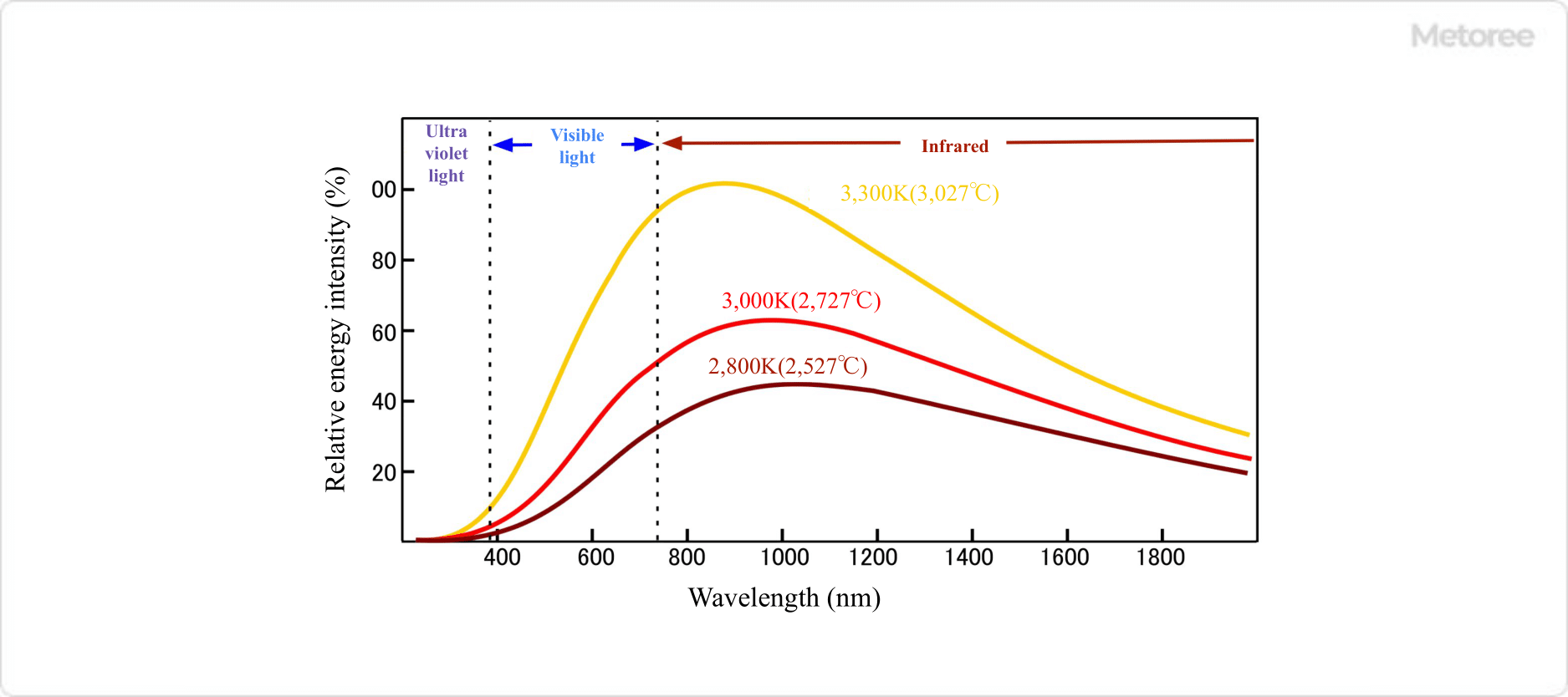
Figure 3. Filament Temperature and Intensity Distribution of Emission Spectrum
The light spectrum emitted from a halogen lamp is almost identical to the blackbody radiation spectrum, which corresponds to the temperature of the filament. Because the temperature of the filament is lower than that of the sun, the emitted light contains almost no ultraviolet light, and its visible light portion has a slightly higher red component, resulting in a warm white light appearance.
The peak of the radiation is in the near-infrared region with wavelengths of 900 to 1,000 nm, and the majority of the radiation is in the visible to near-infrared region with wavelengths of 500 to 3,000 nm.
Compared to ordinary incandescent lamps, halogen lamps allow a smaller distance between the filament and the encapsulated glass. Also, the temperature of the filament can be higher, which offers various advantages.
*Including some distributors, etc.
Sort by Features
Sort by Area

The David Round Company, Inc. was founded in 1869 and is based in Streetsboro, Ohio. The company is one of the world’s oldest hoist manufacturers. The company’s customers include Pfizer, General Mills, Ford, and Exxon Mobile, representing pharmaceutical, food processing, automotive, and oil & gas industries. The company’s custom-engineered hoists and related products include jib cranes, winches, and tractor drives. The company custom engineers machines and replacement parts to fit individual customer needs for projects both large and small.

Larson Electronics, founded in 1973 with headquarters in the USA, is a manufacturer of lighting, electrical control, and explosion-proof equipment. The company's products include explosion-proof lights, corrosion-resistant luminairies for marine use, fishing lights, high-powered stadium lights, and portable power distribution systems for general area and hazardous area use. Larson Electronics' products are used in various industries, including the government, military, aviation, manufacturing, and marine markets. Examples include stadium lights, deep mining lights, lighting used on deep sea oil rigs, and safety lighting in factories.

Bulbrite, founded in 1971 and headquartered in New Jersey, USA, is a manufacturer of lighting products such as string bulbs and LEDs for elegant aesthetics. The company offers a broad assortment of lighting products, including LED bulbs, incandescent bulbs, halogen bulbs, and specialty lamps. The products are designed to provide energy-efficient and optimal lighting solutions for various applications. It emphasis on uniqueness and sustainability ensures that the products deliver benefits such as energy savings, longer lifespan, and reduced environmental impact. These lighting bulbs find applications in residential, commercial, and industrial spaces, enhancing aesthetics, functionality, and energy efficiency.

International Light Technologies was established in 1965 as a manufacturer of light-measuring instruments based in Peabody, Massachusetts. The company provides light measurement products that include light meters, spectrophotometers, radiometers, and spectroradiometers for applications that require measuring of UV, visible light, and of infrared. There are also other accessories such as detectors, filters, calibrations, and integrating spheres that are used along with the main products. The products can withstand harsh environments such as underwater applications and have extensive use in medical, scientific, and industrial manufacturing processes.

Bulbs.com, established in 1999 and headquartered in Worcester, Massachusetts, is an online distributor of various bulbs, serving over 185,000 businesses in over 300,000 locations. Its warehouse stocks over 4,500 replacement bulbs, ballasts, and fixtures from several lighting manufacturers, including Philips, Maxlite, and Bulbrite. The company has been in the lighting industry for over 18 years and sells several other products like electric vehicle chargers, hand tools, and air quality products. It serves several industries, including hospitality, retail, and government sectors, and besides shipping within North America, it can also ship to international addresses.

Allied Electronics Inc., founded in 1991 with headquarters in the USA, is a distributor and supplier of fuel and vehicle servicing station equipment and accessories. The company's extensive product line of over 57,000 products includes forecourt controller parts, fuel dispenser parts, air compressors, and power conditioners. The company also provides rebuilt parts by brands that include Bennett, Daktronics, FE-Petro, and Gasboy. The company's services include equipment financing, primarily serving the retail petroleum industry, including car wash and price sign manufacturers.

StellarNet, Inc. is an American miniature spectrometer system products manufacturer and supplier that was established in Tampa, Florida, in 1991. The company’s product lineup includes portable and compact fiber optic spectrometers for near-infrared (NIR), visible (VIS), and UV measurements. It also offers various specialized light sources such as deuterium, tungsten halogen, and mercury argon. The company’s products are commonly used in academic and research institutions, as well as in the medical industry.

Rhenium Alloys, Inc. is a manufacturer and supplier of high temperature products and refractory metals that was established in 1966 in Elyria, Ohio, USA before relocating to North Ridgeville, Ohio, in 2008. The company offers molybdenum products such as MOCBD heater filaments and flat wire, and rhenium products such as perrhenic acid and zone refined materials. It also offers products made from pure tungsten or tungsten alloys, including rods, sheets, and plates. The company mainly serves clients in the aerospace, defense, and energy industries.

L.A. Woolley Electric, Inc. (L.A. Woolley) was founded in Buffalo, New York in 1916 as a supply distributor to serve the industrial and commercial marketplace. The company offers products from such manufacturers as Acme, Mersen, Pass & Seymor, and Wago. The company specializes in control and panel board products, transformers, terminal blocks, wiring accessories, as well as hazardous location products. The company's in-house sales team also support customers in selecting, ordering, and receiving the correct order.

North American Signal manufactures truck, auto, and industrial warning lights and accessories. Established in 1962 by Julius Newman and partners, NASAG was originally based on North Pulaski Road, Chicago, but is now based in Wheeling, Illinois, with a 45,000-square-foot warehouse. From LEDs, strobe, and battery-powered portable lights to revolving and specialty options, NASAG designs and manufactures warning lights to suit nearly any application. Besides truck and automotive, North American Signal Company also serves industrial markets such as construction and law enforcement.

Orr & Orr, Inc. has been offering wholesale distribution services since 1934 out of Bedford Park, IL. Orr & Orr emphasizes speed of order processing, shipping, and both customer and distributor satisfaction. Product categories that Orr & Orr offers include air conditioners, brake controls, cargo restraint equipment, door tracks, lighting equipment, fans, gas springs, heating equipment, hinges, plumbing supplies, seat belts, steps, toolboxes, hitch and towing products, various truck hardware, cables, battery products, safety equipment, and spring latches.

Kahoku Lighting Solutions Corporation, founded in 1927, is a manufacturer and supplier of lamps such as reflector lamps, Xenon/Xenon mercury lamps, LED lamps, metal halide lamps, and single-ended lamps. The company in Miyagi-ken, Japan has sales office in Saitama-ken Japan and in 2012 the company opened a factory in Binh Duong Province, Vietnam. Kahoku Lighting Solutions is ISO 9001 and ISO 14001 certified. The lamps are used in optical fiber for the inspection process, medical equipment, semiconductor manufacturing equipment, studio lighting, and light distribution analyzer.


PIAA Corporation, established in 1963 and headquartered in Bunkyo Ward, Tokyo, is a manufacturer of automobile supplies and parts. The company produces automotive parts, including bulbs, lamps, and wipers, as well as LED and halogen lighting technologies for night-riding visibility, serving the motorcycle and automotive sectors. It offers visibility products, such as silicone wiper blades and light bars for vehicles. The company also caters to the motorcycle industry with LED and halogen lighting products for night-riding visibility. Its technology extends to ATVs and UTVs with the addition of sports horns for off-road safety.

LEDVANCE, established in 2016, is located in Garching, Germany, and is a manufacturer and supplier of advanced lighting solutions. The company's extensive product range includes a wide variety of LED lighting products, encompassing bulbs, tubes, panels, and luminaires. These products cater to diverse lighting needs in commercial, industrial, and residential spaces. LEDVANCE's lighting solutions provide energy-efficient illumination with adequate quality and longevity. They play a vital role in enhancing visibility, ambiance, and productivity across different environments. The company empowers customers with cutting-edge lighting technology that reduces energy consumption and environmental impact while delivering convenient lighting experiences.

Phillips has been a manufacturer of lighting products since 1891 and is headquartered in Eindhoven, Netherlands. The company is known for its lighting applications with products that include Indoor and outdoor luminaires, LED electronics, lamps, and tubes, along with Conventional lamps and tubes, and lighting controls. Their use expands to offices and industries for lighting indoors, parking lots, healthcare facilities, and public spaces such as airports, parks, plazas, recreational spaces, tunnels, etc. Even the retail and hospitality departments avail the services such as convenience stores along with Horticulture and Aquaculture sectors as well.

Through its subsidiaries, Heraeus Group serves many industries around the world including green power generation, telecommunications, agriculture, aerospace, medical, jewelry, and environmental protection. Heraeus Group companies create products including fiber optics, ceramics, custom metals, surgical equipment, special purpose lamps, and photovoltaics.

Visual Comfort & Co., founded in 1987 and headquartered in Houston, Texas, is a manufacturer of lighting fixtures for the home decor and lighting industry with offices and showrooms across the United States. The company partners with a growing list of designers to create handmade, limited collections of high-end products, including bathroom lighting fixtures, ceiling fans, and floor lamps. Its outdoor products include step lights, gas lanterns, and wall lights. The company's products are available to individual consumers and trade partners, and it also works with clients to provide unique collections for commercial projects.

Litetronics International, Inc. is a UL, Energy Star, and DLC-certified manufacturer of LED lighting products established in 1970 and based in Bedford Park, Illinois, USA. The company offers LED luminaire fixtures for commercial or residential applications, LED retrofit kits for upgrading existing lighting systems, and LED high bays for industrial facilities or spaces with high ceiling overhead. It also offers custom design and production services for unique projects. The company chiefly serves clients in the commercial, retail, and financial sectors.
P.Q.L. Inc. (Premium Quality Lighting), established in 1989 and headquartered in California, United States, is a manufacturer and distributor of lighting products and systems. The company provides various solutions for architectural lighting, and its products include LED cooler lights, LED outdoor fixtures, and emergency backup power systems. Its products are used in residential, commercial, and industrial situations. It primarily serves retailers and wholesale buyers through its distribution centers located across the United States. It also provides original equipment manufacturing and private label production services.

Meiji Techno Co., Ltd. (MTC) is a Japanese manufacturer and distributor of optical microscopes originally established in 1964 as Azuma Optics Company before rebranding in 1975. Based in Saitama, the company produces various microscopes for applications in education and life sciences, including inverted, compound, stereo, and polarizing models. These are utilized by clients in laboratory operations and quality control, analysis, and testing within the industrial sector. MTC has manufacturing facilities in Japan and China, with distribution operations in Shanghai, California, and New Jersey. Its North American subsidiary Meiji Techno America was incorporated in 1986, and is located in San Jose.

TPI Corporation is a manufacturer of HVAC and lighting products that was founded in 1950 and based in Johnson City, Tennessee, USA. The company produces hardware used for ventilation and heating, as well as specialized ovens and commercial controls. These products include air curtains, industrial ovens for curing operations with various materials, and hazardous location lighting products. The company primarily serves customers in the commercial and industrial sectors, but does offer products used by homeowners and residential contractors.

Ushio America, Inc., established in 1967 and headquartered in Cypress, California, is a manufacturer and distributor of photonics solutions. Its diverse product range includes xenon short arc, lasers, ultra-high-pressure UV, excimer, and metal halide lamps, serving various industries such as biotechnology, electronics, medical, and semiconductor. The company has obtained multiple certifications, including ENERGY STAR, DesignLights Consortium, and Underwriters Laboratories Inc. Its products are designed and manufactured in an ISO 13485-certified medical facility.


Princess Auto Ltd., founded in 1933 and based in Winnipeg, Canada, is a supplier of an assortment of tools and equipment with over 50 stores across Canada. In 1977, it opened its first store in Edmonton, Alberta, and became a retailer. The company serves several workers, including inventors, farmers, and tradespeople and stocks products such as outdoor power equipment, hand tools, and hydraulics. It sells items from several brands, such as Dealer’s Choice, Braber Equipment, and King Canada, and it also has a foundation that provides financial assistance to trades students enrolled at Canadian colleges.

Hello BOM is an online electronic component storefront designed to bridge customers and suppliers. Customers supply Hello BOM with their bill of materials (BOM) and Hello BOM uses smart analysis via AI data matching to quickly find precise matches that customers need. This is accomplished via Hello BOM’s growing component stock and product information database to grow increasingly accurate and fast, while also simplifying the process of R&D and purchasing for customers. Products include semiconductors, resistors, capacitors, diodes, inductors, connectors, transistors, and sensors.
Ranking as of July 2025
Derivation Method| Rank | Company | Click Share |
|---|---|---|
| 1 | The David Round Co., Inc. |
10.4%
|
| 2 | Bulbrite |
8.7%
|
| 3 | Heraeus Holding GmbH |
8.7%
|
| 4 | International Light Technologies, Inc. |
6.8%
|
| 5 | Larson Electronics, LLC |
6.1%
|
| 6 | Meiji Techno |
5.5%
|
| 7 | Signify Holding |
5.2%
|
| 8 | L. A. Woolley Electric, Inc. |
4.2%
|
| 9 | Kahoku Lighting Solutions Corporation |
4.2%
|
| 10 | PIAA Corporation |
4.2%
|
Derivation Method
The ranking is calculated based on the click share within the halogen lamp page as of July 2025. Click share is defined as the total number of clicks for all companies during the period divided by the number of clicks for each company.Number of Employees
Newly Established Company
Company with a History
*Including some distributors, etc.
*Including some distributors, etc.
| Country | Number of Companies | Share (%) |
|---|---|---|
 United States of America
United States of America
|
11 | 64.7% |
 Japan
Japan
|
3 | 17.6% |
 Germany
Germany
|
2 | 11.8% |
 Netherlands
Netherlands
|
1 | 5.9% |
93 products found
93 products
Life Elex Co., Ltd.
990+ people viewing
Last viewed: 6 hours ago
The sealed halogen gas maintains the original brightness until the end of its life without any blackening phenomenon. We offer a wide variety of pr...
10 models listed
Life Elex Co., Ltd.
780+ people viewing
Last viewed: 7 hours ago
This JC is widely used in optical equipment and measurement equipment, and has a proven track record and reliability.
3 models listed
FujiLamp
230+ people viewing
Last viewed: 10 hours ago
Fintech Co., Ltd.
270+ people viewing
Last viewed: 1 day ago
The light source (filament) reaches temperatures of 2,500℃ to 3,000℃. If this light is concentrated well, clean heating can be achieved without con...
FujiLamp
190+ people viewing
Last viewed: 6 hours ago
Yagyu Shokai Co., Ltd.
210+ people viewing
Last viewed: 8 hours ago
■Features ・The light guide is flexible and can be illuminated at any position. -Uses a high-brightness, long-life halogen lamp that will not deter...
Yagyu Shokai Co., Ltd.
200+ people viewing
■LA-150UE - Type with light guide. -Uses low-noise, long-life halogen lamps. - Boasts high illuminance with low voltage. ・Safe design allows conti...
Novitec Co., Ltd.
280+ people viewing
Last viewed: 12 hours ago
This is a halogen type lighting. It can illuminate a wide area and is relatively inexpensive, making it suitable for high-speed photography of mech...
Optar Co., Ltd.
230+ people viewing
Last viewed: 1 day ago
Halogen lamps can emit a wide range of light from visible light to infrared light.
Hakuron Seisakusho Co., Ltd.
270+ people viewing
■Characteristics Because halogen lamp heaters heat directly without a heat medium, they are capable of stable, completely clean heating and heating...
Unisoku Co., Ltd.
160+ people viewing
Last viewed: 3 hours ago
Taikatsu Sangyo Co., Ltd.
280+ people viewing
Last viewed: 16 hours ago
■Features ・Adopts an aluminum body with excellent impact resistance and durability ・With heat-resistant full glass ・Safe with guard ・The light ...
Eiwa Denki Co., Ltd.
180+ people viewing
Last viewed: 1 day ago
Hakuron Seisakusho Co., Ltd.
280+ people viewing
Last viewed: 1 hour ago
When the light from a halogen lamp is focused on a single point, high temperatures reaching 1,400℃ to 1,500℃ can be obtained if conditions are good...
Hakuron Seisakusho Co., Ltd.
210+ people viewing
Last viewed: 16 hours ago
When the light from a halogen lamp is focused on a single point, high temperatures reaching 1,400℃ to 1,500℃ can be obtained if conditions are good...
Daruka Sangyo Co., Ltd.
230+ people viewing
Last viewed: 9 hours ago
■Features ・Irradiation in the near-infrared region is achieved using a dedicated 150W halogen lamp. ・The required peak wavelength for each applic...
FujiLamp
210+ people viewing
Last viewed: 2 hours ago
The ``Long Star'', which has become synonymous with halogen lamps for onboard fishing, has been further improved to create a cost-effective halogen...
FujiLamp
170+ people viewing
Last viewed: 19 hours ago
We have made the long star, which boasts a proven track record, a double glass structure, making it possible to use it underwater. It achieves low ...
Tsubosaka Electric Co., Ltd.
220+ people viewing
Last viewed: 9 hours ago
■Features ・Four color temperatures can be switched by switch operation or external communication ・Outputs highly uniform brightness with a bright...
Tsubosaka Electric Co., Ltd.
230+ people viewing
Last viewed: 2 hours ago
■Features ・Single point light source that outputs stable brightness ・Brightness can be finely adjusted ・Small and lightweight, can be used anywh...
FujiLamp
170+ people viewing
Last viewed: 1 day ago
This is a system that prevents lamp damage due to impact.
Tsubosaka Electric Co., Ltd.
210+ people viewing
Last viewed: 4 hours ago
■Features - Manually adjust the brightness value while looking at the display ・Achieves performance similar to LSB-111BAT2 at a low price ・Color ...
Taikatsu Sangyo Co., Ltd.
220+ people viewing
Last viewed: 46 minutes ago
■Features ・Outdoor use/Rainproof type - Automatically turns on for about 6 seconds to 12 minutes when the sensor detects a person, car, etc. - Aut...
Tsubosaka Electric Co., Ltd.
210+ people viewing
Last viewed: 4 hours ago
■Features ・Multi-point brightness light source that feeds back brightness with switchable color temperature ・Outputs stable brightness with light...