






7 Nickel Metal Hydride Battery Manufacturers in 2024
This section provides an overview for nickel metal hydride batteries as well as their applications and principles. Also, please take a look at the list of 7 nickel metal hydride battery manufacturers and their company rankings. Here are the top-ranked nickel metal hydride battery companies as of April, 2024: 1.Camelion Batterien GmbH, 2.DYNAMIS Batterien GmbH, 3.FDK CORPORATION.
Table of Contents
What Is a Nickel Metal Hydride Battery?

A nickel metal hydride battery is a type of rechargeable battery that can be charged and discharged, using a hydrogen storage alloy for the negative electrode and nickel hydroxide for the positive electrode.
Nickel metal hydride batteries are expensive because they use a hydrogen storage alloy instead of cadmium, but can be charged and discharged using a large current and have a large capacity per unit mass.
In addition, compared to other rechargeable batteries, nickel-metal hydride batteries have a relatively small memory effect (the voltage drop that occurs during discharge when batteries are repeatedly recharged without being fully discharged), and can be used repeatedly without performance degradation.
Uses of Nickel Metal Hydride Batteries
Nickel metal hydride batteries are used to take advantage of their high performance and long life, and are used in automotive batteries, notebook PCs, dry cell batteries, and other applications requiring high output and reliability.
In recent years, lithium-ion batteries, which do not have memory effect or self-discharge and have a larger capacity per unit mass and higher operating voltage, have come to be used.
Principles of Nickel Metal Hydride Batteries
1. Composition of Nickel Metal Hydride Battery
Nickel metal hydride batteries consist of electrodes (positive electrode: nickel oxyhydroxide, negative electrode: hydrogen storage alloy), a separator made of olefin nonwoven fabric, and potassium hydroxide solution as electrolyte. In the case of a dry cell, the structure wound around these components is contained in a can.
2. Charge-Discharge Reaction of Nickel Metal Hydride Battery
During discharge of a nickel metal hydride battery, at the positive electrode, nickel oxyhydroxide receives electrons in the presence of water, producing nickel hydroxide and hydroxide ions. At the anode, hydrogen ions and electrons are released from the hydrogen storage alloy in the presence of hydroxide ions to produce water.
During discharge, the reaction proceeds in the opposite direction: at the cathode, hydroxide ions react with nickel hydroxide to produce nickel oxyhydroxide, which releases electrons. At the anode, hydrogen is adsorbed by supplying electrons.
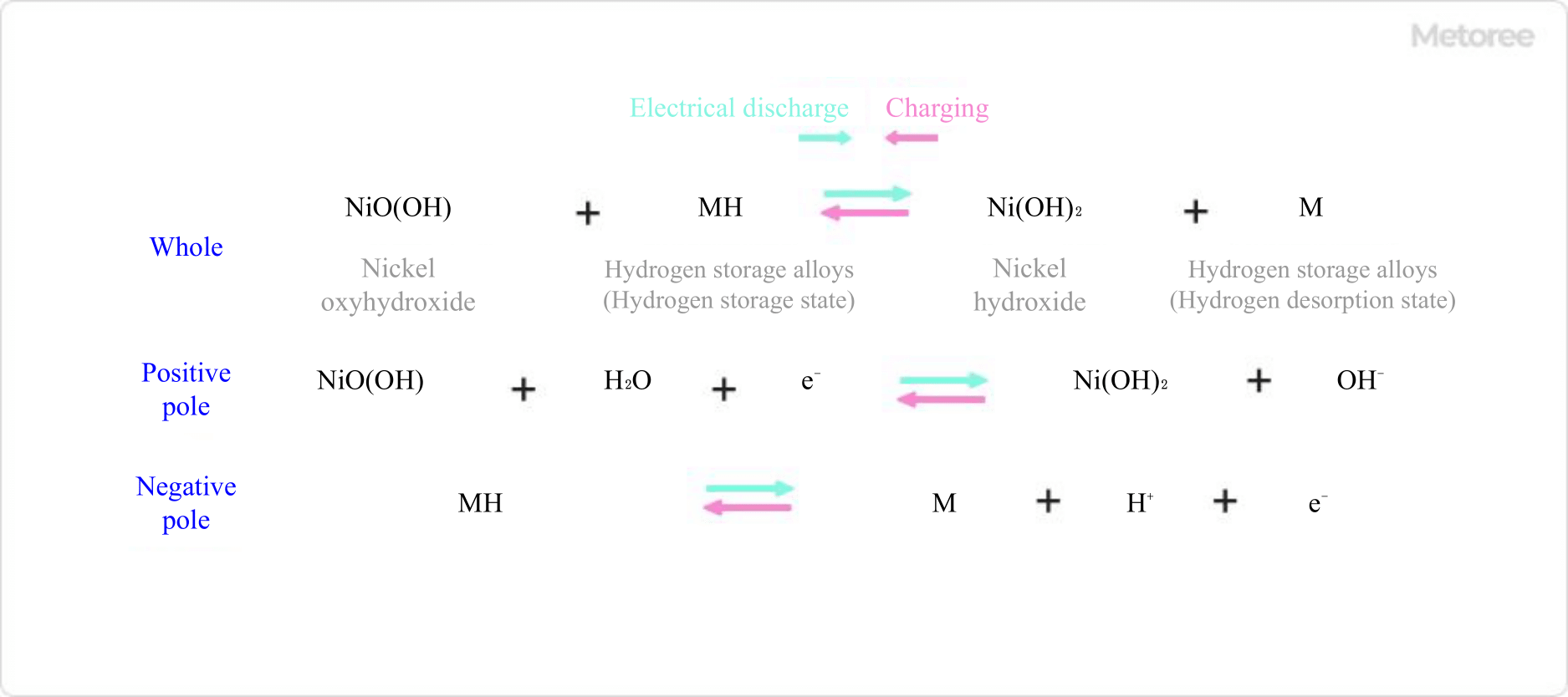
Figure 1. Charge-discharge reaction equation of a nickel-metal hydride battery
The charging and discharging of a nickel metal hydride battery occurs through a simple reaction that involves the adsorption of hydrogen and the production of water. For example, lead-acid batteries used in automobile batteries are charged and discharged through a precipitation-dissolution reaction of the electrodes, so repeated charging and discharging inevitably results in deterioration of the electrodes. A nickel metal hydride battery has no such degradation mode and can be used semi-permanently as long as the electrode itself does not deteriorate, making it a battery with a long service life.
3. Electrodes of Nickel Metal Hydride Battery
Co-alloys have been mainly used for the negative electrode in the past in order to achieve high capacity, but there has been a move toward Co-free electrodes mainly due to cost considerations. However, the use of Co-fewer alloys has been progressing, mainly due to cost considerations. As for the cathode, nickel oxyhydroxide in the charged state is highly conductive, but nickel hydroxide in the discharged state is an insulator, which causes problems such as loss of electron paths during discharge. For this reason, cobalt oxyhydroxide or other materials are added to give conductivity.
Other Information on Nickel Metal Hydride Batteries
Nickel Metal Hydride Battery Characteristics
1. Battery Characteristics
The nominal voltage of nickel metal hydride battery is 1.2V, which is the same as that of a nickel-cadmium battery. This is because the reactions used for charging and discharging are similar. Since the nominal voltage of lead-acid batteries is 2.0 V and the rated voltage of lithium-ion batteries is 3.7 V, they are relatively low voltage batteries. Since these batteries can easily carry a large current, they are used in equipment that requires high output, such as hybrid cars.
Nickel metal hydride batteries have a memory effect (the voltage of the battery drops as it is repeatedly recharged, resulting in a decrease in usable capacity and an inerting effect. Therefore, understanding the characteristics of the battery when using it will maximize its service life.
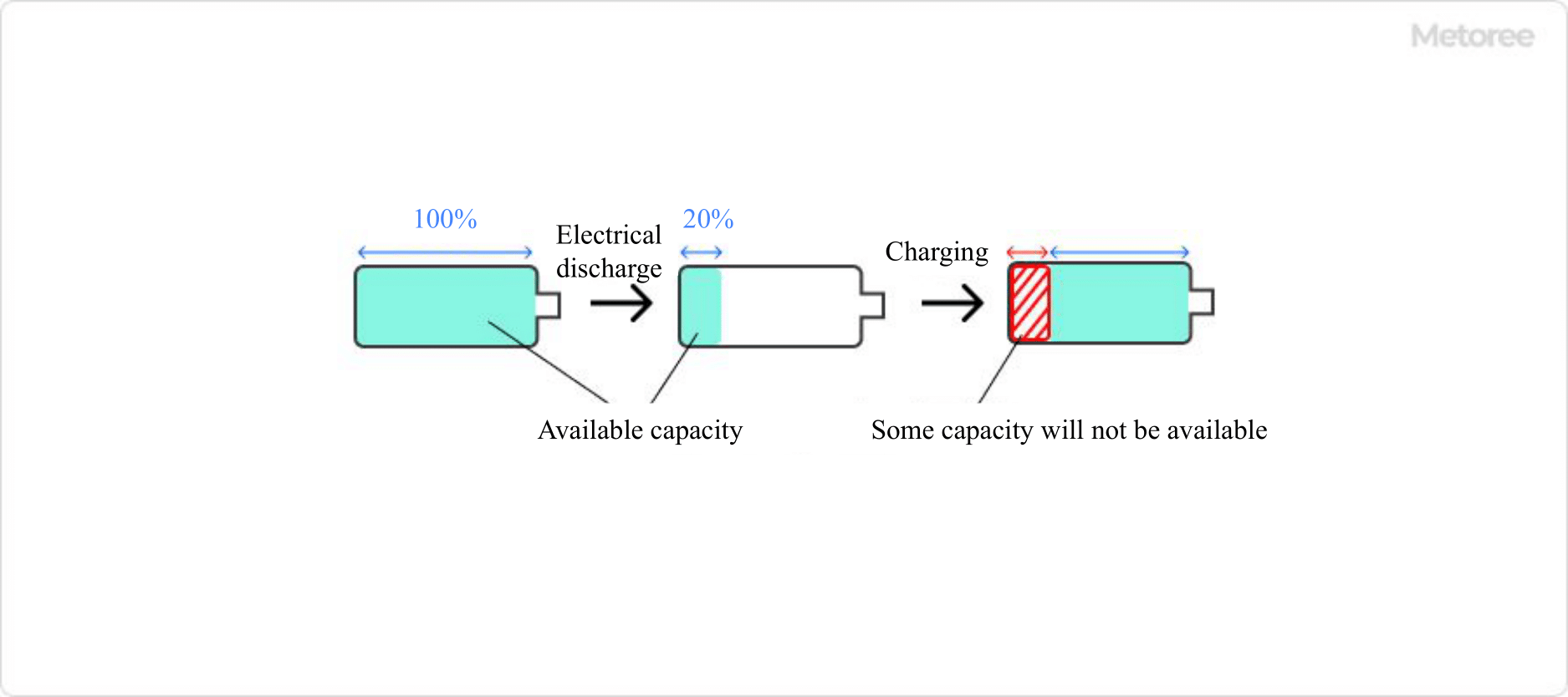
Figure 2. Memory effect
2. Safety
Basically, battery explosions and fires are caused by the ignition of organic solvents, which are electrolyte solvents, by sparks created by short circuits.
The electrolyte solvent in nickel metal hydride batteries is water, so even if a spark should occur, it will not ignite. Therefore, the current and voltage control mechanisms do not need to be designed as rigorously as in lithium-ion batteries, thus lowering the manufacturing cost. This low cost is one of the reasons why nickel metal hydride batteries are still widely used in industry.
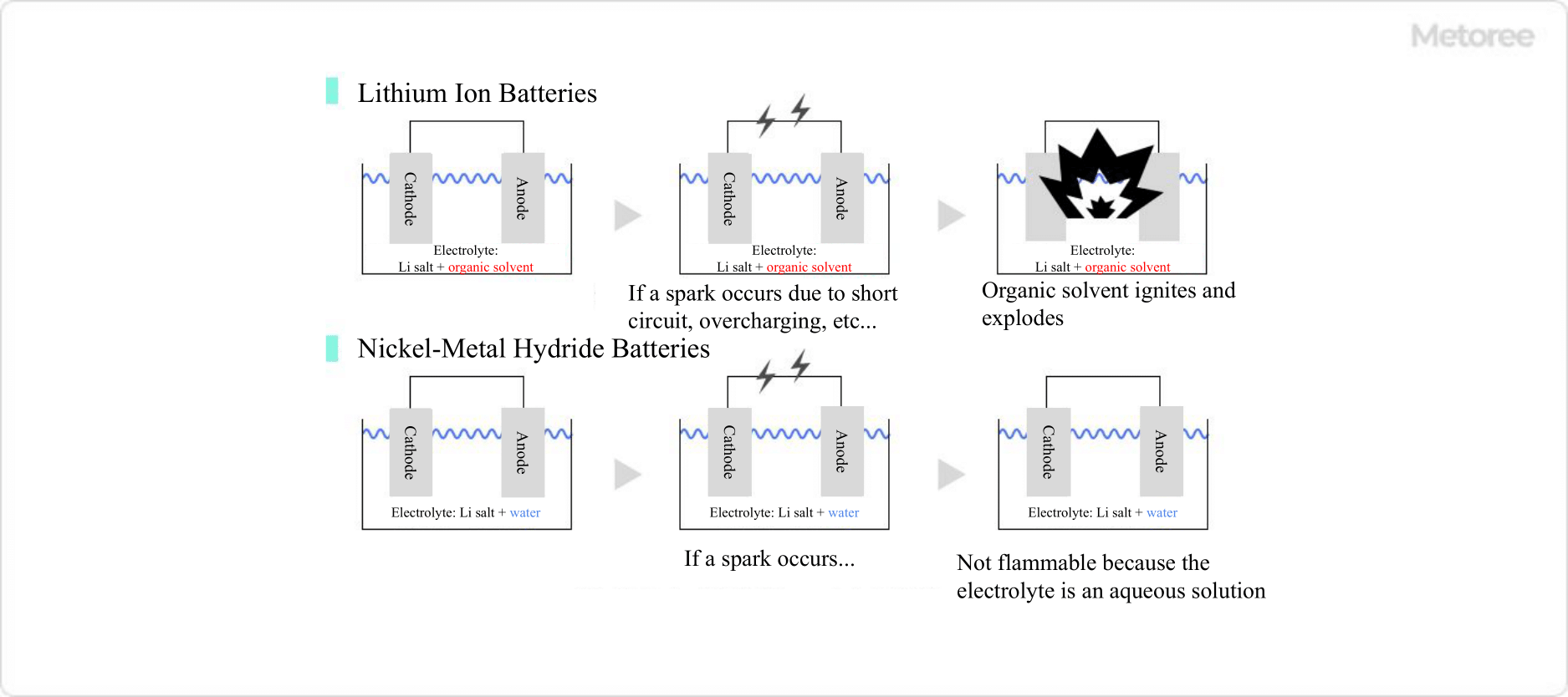
Figure 3. Comparison of lithium-ion and nickel-metal hydride batteries
3. Environmental Impact
Lithium-ion batteries, lead-acid batteries, and nickel-cadmium batteries contain hazardous substances with a high environmental impact (e.g., cadmium in nickel-cadmium batteries is a causative agent of Itai-itai disease, one of the four major pollution diseases). The electrolyte is also an environmentally friendly storage battery because it does not use organic solvents.
List of 7 Nickel Metal Hydride Battery Manufacturers
*Including some distributors, etc.
Sort by Features
- Default
- Company Size: largest first
- Year Founded: oldest first
- Year Founded: earliest first
Sort by Area
- Germany
- Hong Kong
- Japan
-
-

-
DYNAMIS Batterien GmbH
NiMH accus
Manufacturer Overview
GmbH, founded in 2008 and based in Dettingen, Bayern, Germany, is a manufacturer and supplier of quality batteries and power storage solutions. The company's product portfolio includes a wide range of batteries, such as lead-acid batteries, lithium batteries, and gel batteries, designed for various applications like industrial, automotive, and renewable energy storage. Dynamis Batterien's advanced battery technologies ensure good power supply and efficient energy storage, meeting the diverse demands of industries worldwide. With an aim for sustainability, Dynamis Batterien aims to provide cutting-edge solutions that makes a greener and more efficient future.
-
-
-
-

-
Camelion Batterien GmbH
NI-MH rechargeable battery
Manufacturer Overview
Camelion Batterien GmbH was established in 1997 and is based in Berlin, Germany, as a manufacturer of power supply equipment. Its specialization is manufacturing alkaline batteries, rechargeable batteries, chargers, power banks, and flashlights. The company obtained several ISO certifications, including ISO 9001 and ISO 14000. It has partnered with companies such as Westinghouse and Shell. Its manufactured products are catered to the electronics, electrical appliances, automotive, and manufacturing fields across Germany.
-
-
-
-

-
FDK CORPORATION
Ni-MH battery
Manufacturer Overview
FDK Corporation, founded in 1950 and headquartered in Tokyo, Japan, is a manufacturer and supplier of electronic components and batteries. Its diverse product range includes alkaline batteries for devices like toys and remote controls, Ni-MH batteries for eco-friendly rechargeable power, lithium batteries for medical devices and smart meters, carbon-zinc batteries for economical energy, and power supplies encompassing switching units and DC-DC converters. Furthermore, the company has several production plants located all over the globe, such as in Korea, Taiwan, Hong Kong, and Germany.
-
-
-
-

-
GPI International Limited
NiMH
Manufacturer Overview
GP Energy Tech, established in 2022 in China and Singapore, is a developer and manufacturer of energy storage solutions. The company specializes in rechargeable battery design and manufacturing, aimed at sustainability and cutting-edge technologies. Their high-performing batteries find applications in electric vehicles, renewable energy, consumer electronics, and specialized industries. GP Energy Tech prioritizes eco-friendly materials and processes to minimize environmental impact. With a heritage spanning six decades through GP Batteries, they leverage expertise and global reach to drive the transition to sustainable energy storage, ensuring a cleaner and more accessible world for the future generation.
-
-
-
-

-
Jauch Quartz
NiMH battery
Manufacturer Overview
Jauch Group, established in 1954 and based in Villingen-Schwenningen, Germany, is a manufacturer and supplier of quartz crystals, crystal oscillators, and battery technology. The company’s products are used in various applications, including frequency control components for electronics and battery solutions for diverse industries. The company offers in-depth technical consulting, certification expertise, and fast product delivery. With production facilities in Germany and strategic partnerships in Asia, the company ensures the availability of frequency control products. Besides, the company provides new technology solutions and great customer support, catering to the evolving needs of its clients.
-
-
-
-

-
Toshiba Lifestyle Products and Services Corporation
Battery
Company Overview
Toshiba Lifestyle Products & Services Corporation, located in Kawasaki City, Kanagawa, Japan, has been a manufacturer and distributor of household appliances since 1875. The company offers a diverse range of home appliances, including refrigerators, microwaves, vacuum cleaners, irons, and rice cookers, along with washing machines, and air conditioners. With seven domestic group companies and nine overseas bases in Asia, it also operates five manufacturing plants and four sales companies in Thailand, China, Vietnam, Malaysia, and Hong Kong. The company distributes its products and services to customers worldwide.
-
-
-
-

-
VB power
Nickel metal hydride Ni-MH battery
Distributor Overview
VB Power, established in 2008 by Mr. Zhang Wei and based in Dongguan City, Guangdong Province, China, is a supplier of power batteries. The company's product range encompasses NiMH battery series( SC, C, A, 2/3A, 4/5A) and Li-ion battery series (Lipo and LifePO4 battery) with high power and endurance to cryogenic temperature. The products are used in various industries, such as electric motors, cordless power tools, R/C cars and Hobbies, electric airsoft guns, AEG guns, and vacuum cleaners.
-
-
Nickel Metal Hydride Battery Manufacturer Ranking
*Including some distributors, etc.Ranking as of April 2024
Derivation Method| Rank | Company | Click Share |
|---|---|---|
| 1 | Camelion Batterien GmbH |
20.0%
|
| 2 | DYNAMIS Batterien GmbH |
17.6%
|
| 3 | FDK CORPORATION |
15.1%
|
| 4 | GPI International Limited |
13.2%
|
| 5 | Jauch Quartz |
9.5%
|
| 6 | RPC Corporation |
8.4%
|
| 7 | VB power |
8.1%
|
| 8 | Toshiba Lifestyle Products and Services Corporation |
8.1%
|
Derivation Method
The ranking is calculated based on the click share within the nickel metal hydride battery page as of April 2024. Click share is defined as the total number of clicks for all companies during the period divided by the number of clicks for each company.Number of Employees
- FDK CORPORATION: 2,436
Newly Established Company
- Jauch Quartz: 1954 (70 years ago)
Company with a History
- Jauch Quartz: 1954 (70 years ago)
Global Distribution of Nickel Metal Hydride Battery Manufacturers by Country
*Including some distributors, etc.
| Country | Number of Companies | Share (%) |
|---|---|---|
 Germany
Germany
|
3 | 60.0% |
 Japan
Japan
|
1 | 20.0% |
 Hong Kong
Hong Kong
|
1 | 20.0% |