All Categories
History
This section provides an overview for lithium aluminum hydride as well as their applications and principles. Also, please take a look at the list of 0 lithium aluminum hydride manufacturers and their company rankings.
Table of Contents
Categories Related to Lithium Aluminum Hydride
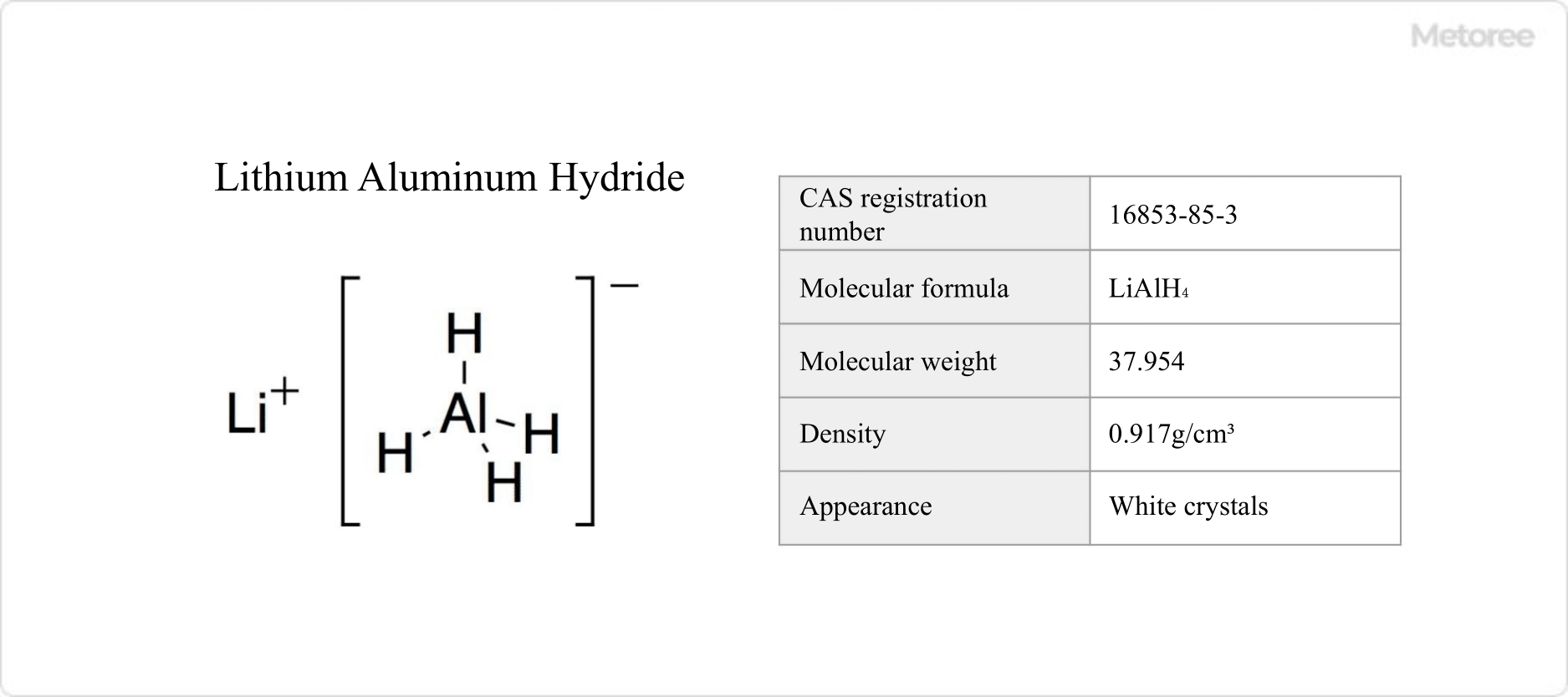
Figure 1. Basic information on aluminum lithium hydride
Lithium aluminum hydride, represented by the chemical formula LiAlH4 and commonly known as LAH, is an inorganic compound with strong reducing properties.
Compared to sodium borohydride (NaBH4), another well-known reducing agent, lithium aluminum hydride has a stronger reducing power and can reduce carboxylic acids and amides.
Due to its high reactivity, lithium aluminum hydride easily ignites and reacts explosively with water, necessitating careful handling.
Lithium aluminum hydride has a strong reducing effect and is a reducing agent in organic chemistry.
For example, it is used to reduce esters, ketones, and aldehydes to alcohols; amines from amides, nitriles, and nitro compounds; and dehalogenated alkanes from halogenated compounds.
Due to its high reactivity, it is not used in large quantities but is often used in small-scale experiments.
Lithium aluminum hydride is a strong reducing agent in powder form. It is much stronger than the reducing agent NaBH4 because the Al-H bonds are weak compared to the B-H bonds.
When lithium aluminum hydride comes into contact with water, it reacts violently, producing hydrogen gas. Therefore, a dehydrating solvent such as diethyl ether is required for use.
It decomposes at 150 °C and has a molar mass of 37.954 g/mol and a density of 0.917 g/cm3.
Lithium aluminum hydride is a hydrogen compound of aluminum, comprising an ionic crystal of aluminum hydride ions (AlH4-) and lithium ions (Li+).
The crystal structure belongs to the monoclinic system, with AlH4- having a tetrahedral structure and an Al-H bond distance of approximately 1.55 Å in the crystal.
Lithium aluminum hydride can be synthesized by the reaction of aluminum chloride (AlCl3) with lithium hydride (LiH). The yield by weight is 97%. The reaction mixture is dissolved in ether and filtered to remove the remaining solid lithium chloride (LiCl).
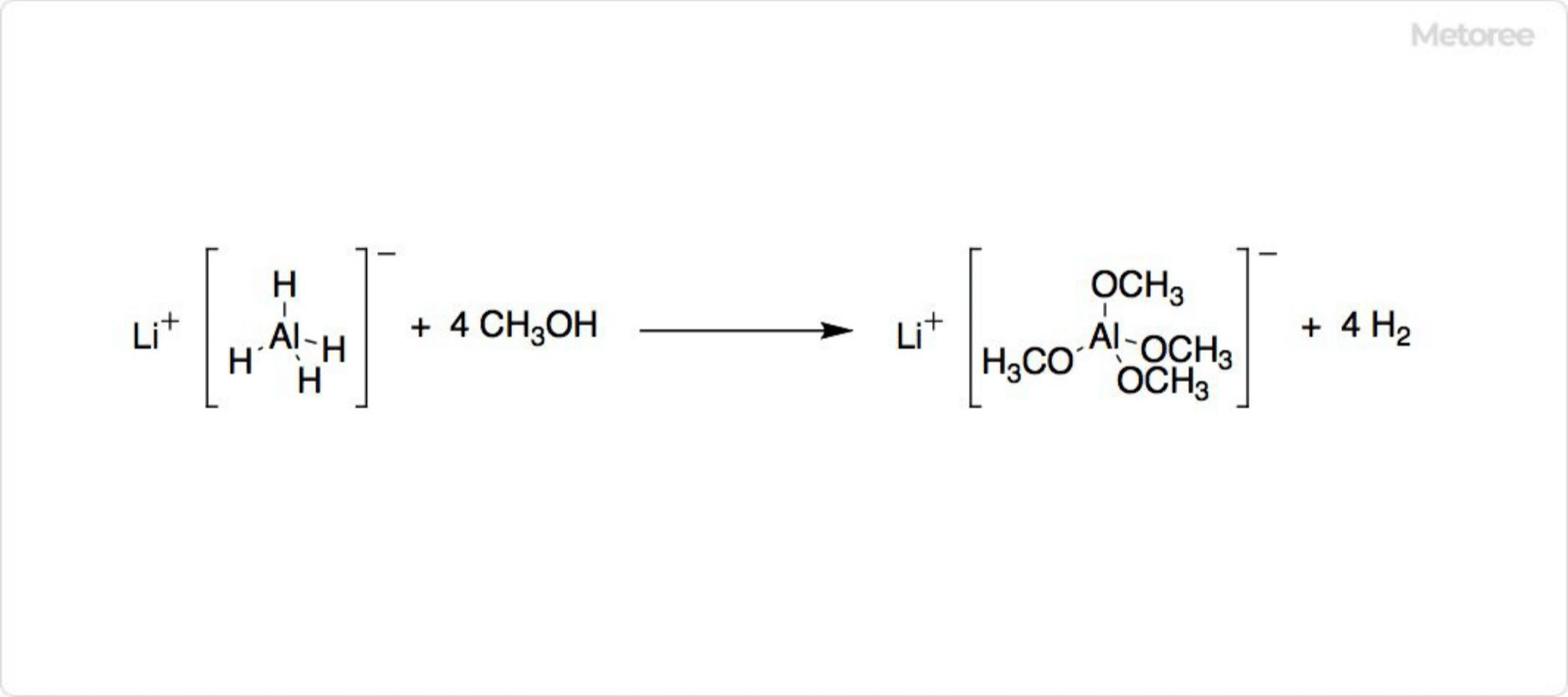
Figure 2. Decomposition of lithium aluminum hydride
Lithium aluminum hydride is strongly basic and decomposes in a single reaction with a protic solvent such as alcohol.
At room temperature, lithium aluminum hydride is metastable.
When stored for long periods, especially when stored for extended periods, lithium aluminum hydride gradually decomposes into LiH and Li3AlH6. The presence of iron, titanium, vanadium, etc. accelerates the decomposition.
Lithium aluminum hydride reacts with sodium hydride in THF to form sodium aluminum hydride (NaAlH4). Similarly, potassium aluminum hydride (KAlH4) can be synthesized in 90% yield.
The reverse reaction of NaAlH4 and KAlH4 to lithium aluminum hydride also proceeds with LiCl using THF or diethyl ether as a solvent.
Otherwise, lithium aluminum hydride reacts with magnesium bromide (MgBr2) to magnesium aluminum hydride (Mg(AlH4)2).
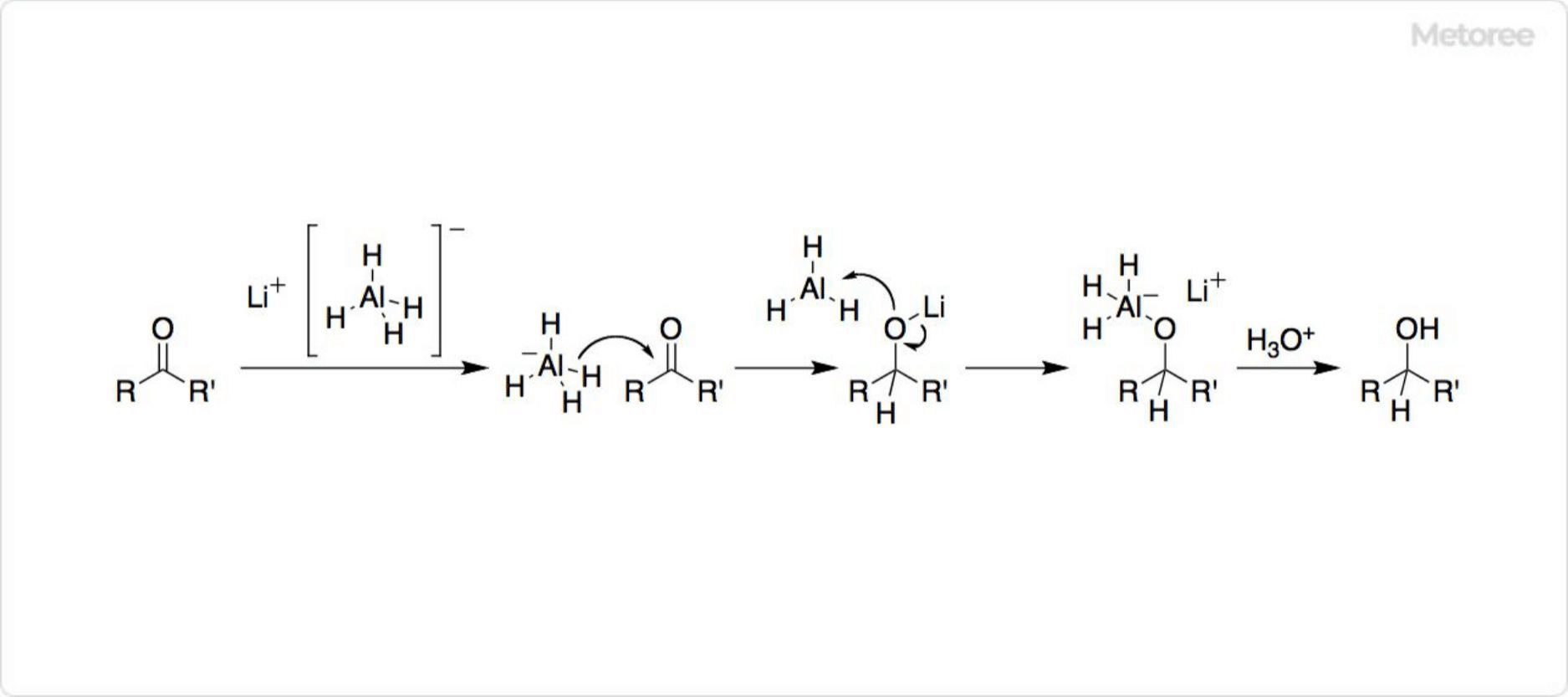
Figure 3. Reaction mechanism of lithium aluminum hydride
Lithium aluminum hydride is highly reactive and can be a powerful reducing agent in organic chemistry. In particular, the reduction of esters and carboxylic acids to primary alcohols is widely known.
In this reaction, hydride ions (H-) react with the active center of organic compounds with low electron density by the inductive effect and mesomeric effect simultaneously with the decomposition of AlH4-. The reaction is called the inductive effect.
*Including some distributors, etc.
Sort by Features
Number of Employees
Newly Established Company
Company with a History